The following content is excerpted from the Prospectus of Titan Mining Corporation dated August 18, 2017 filed on SEDAR.
General Overview
Zinc is one of the most widely used base metals in the world, known for its ability to resist
oxidation and corrosion. The metal appears a lustrous bluish-white and holds a number of
beneficial physical, electrochemical and chemical properties that allow for it to be used
in a variety of applications. Zinc is chemically active and alloys readily with other metals
such as copper, aluminum and magnesium. Zinc also reacts readily with iron when used for
galvanizing, which imparts desirable characteristics such as strong corrosion resistance and
high durability. Zinc is a relatively hard metal with a low melting point, making it and its
alloys ideal for die casting while remaining malleable enough to be formed, rolled or extruded,
according to the
International Zinc Association (IZA).
According to the IZA, zinc is the 23rd most abundant element contained within the earth's crust
(averaging 70 ppm) and is mined in over 50 countries worldwide. Zinc ore bodies are formed in
various tectonic environments and at different temperatures and pressures, which causes
significant variability in the properties of ore bodies found across the world. Eight main ore
body classifications exist to account for this variability: sedex, volcanogenic massive sulfide
("VMS"), oxide, carbonate replacement, Mississippi valley type ("MVT"), epithermal and
mesothermal and other miscellaneous hydrothermal. According to the IZA, the most prevalent of
these deposits are sedex, which are critical sources of global zinc supply. Sedex deposits are
typically found in sedimentary rocks, particularly shales, with mineralization that exceeds 100 Mmt
and with grades of 10% to 20% Zn. VMS deposits are also common and widely distributed across
the globe. They tend to be smaller than sedex deposits but often have a higher by-product
presence (copper, lead and silver). MVT deposits tend to be smaller and lower grade (2% to 6%),
but often occur in clusters within a single district. Few oxide deposits of zinc are known.
Zinc ore is generally extracted as a co-product with other metals, most notably copper, lead,
gold and silver. Zinc ores commonly contain approximately 5% to 15% Zn and are most commonly
found as the zinc mineral sphalerite, also known as zinc blende. Zinc blende accounts for
over 95% of zinc produced globally. According to the IZA, zinc production is sourced from both
underground and open pit operations. On a production volume basis, the IZA estimates that
underground operations account for 64% of global production, combined underground and open
pit operations account for 21% and open pit operations account for 15%.
Once ore is extracted, it is crushed and ground to separate it from other minerals, and is then
typically concentrated using the froth floatation method. The IZA estimates that this yields a
zinc concentrate containing between 45% and 55% Zn. According to the IZA, the contained zinc
can be recovered using either pyrometallurgical or hydrometallurgical techniques, though roughly
90% of zinc is produced using the latter method. The zinc produced by the hydrometallurgical
process has the advantage of being high grade (99.99% Zn), whereas a further distillation
process is needed to achieve this grade from the pyrometallurgical technique. However,
the pyrometallurgical technique may be used on a wide range of materials, particularly mixed
zinc-lead bulk concentrates. Zinc metal is marketed in three grades depending on its purity:
Special High Grade (99.995% Zn), High Grade (99.99% Zn) and Good Ordinary Brand (98.5% Zn).
Zinc Supply and Uses
Click here to see the latest ILZSG (The International Lead and Zinc Study Group) monthly data on zinc supply and demand
| Global Top 20 Producing Primary Zinc Mines by Zinc Reserve Grade |
| Perkoa, Burkina Faso (Trevali) | 15.2% |
| Red Dog, Alaska, USA (Teck) | 15.0% |
| Rampura Agucha, India (Hindustan) | 13.9% |
| Kayar, India (Hindustan) | 13.4% |
| Illapa, Bolivia (Combiol / Glencore) | 10.1% |
| Jaguar, Western Australia (Independence) | 9.5% |
| McArthur River, Australia (Glencore) | 9.4% |
| Empire State Mine, New York, USA (Titan) | 9.2% |
| Skorpion, Namibia (Vedanta) | 9.0% |
| San Vicente, Bolivia (Minera San Ignacio) | 8.9% |
| Rosh Pinah, Namibia (Glencore) | 8.8% |
| Langlois, Quebec, Canada (Nyrstar) | 8.6% |
| Rosebery, Australia (MMG) | 8.3% |
| Mt Isa Zinc, Australia (Glencore) | 8.2% |
| Pend Oreille, Washington, USA (Teck) | 8.1% |
| Sinchi Wayra, Bolivia (Glencore) | 8.1% |
| Aguilar, Argentina (Glencore) | 7.6% |
| Santa Eulalia, Mexico (Southern Copper) | 6.9% |
| Matagami, Quebec, Canada (Glencore) | 6.8% |
| Rajpura-Dariba, India (Hindustan) | 6.3% |
| Tara, Ireland (Boliden) | 6.3% |
|
Supply
According to the January 2016 United States Geological Survey (the "USGS") Zinc Commodity
Summary, the vast majority of global zinc supply is sourced from China, though Australia and
Peru are meaningful producers as well. According to the USGS, many of the world's largest zinc
mines are located in Latin America and the United States, though the largest zinc mineral
reserves are in Australia, China and Peru.
As all mines have finite lifespans, new projects must be constantly identified and developed in
order to maintain balance in the global zinc markets. However, determining whether or not an
identified project can be advanced to production is very complex. While many zinc deposits have
been identified as potential sources of future supply, the decision to proceed with production
requires the completion of significant geological, financial and environmental diligence. Due
to these extensive diligence requirements, new mine production typically requires long lead
times.
Commodity fundamentals can also have an impact on currently producing operations. For example,
as a result of thin zinc margins through 2015, on October 9, 2015, Glencore PLC announced a 500 kmt
per annum cut to zinc production. This represented approximately 4% of refined zinc metal
production in 2015, based on the ILZSG global supply estimate for 2015.
Uses
| First Uses of Zinc |
| Galvanizing | 50% |
| Die-Casting Alloys | 17% |
| Brass & Bronze | 17% |
| Zinc Semi-Manufacturing | 6% |
| Chemicals | 6% |
| Misc. | 4% |
|
Source:
The International Lead and Zinc Study Group (ILZSG)
According to the ILZSG, galvanizing is the predominant first-use for zinc due to its ability to
provide a protective barrier against corrosion. Galvanized steel is most commonly used in the
automotive industry where it permits automakers to reduce costs and body weight without
compromising safety or appearance. Outside of the automotive industry, the alloys Galfan and
Galvalume are used in niche markets. Galvalume, a zinc-aluminum alloy comprised of 45% zinc and
55% aluminum, is used in construction to improve corrosion resistance of unpainted surfaces.
Galfan is a similar zinc-aluminum alloy, however, it has an additional 5% of aluminum.
A benefit of the additional aluminum content is that it has improved formability, making it
more useful for architectural purposes than other zinc coatings. While galvanizing has long
comprised the largest first use consumption of zinc, China Minmetals Corporation has stated
that it has also been the fastest growing area of zinc consumption, heavily driven by China's
infrastructure, power and communication boom.
According to the IZA, die-casting alloys are used extensively in the automotive and residential
construction sectors with growing demand for use in business machines, toys, bathroom fittings,
electrical appliances and domestic appliances. According to the IZA, zinc alloys have
traditionally been preferred due to their strength, innate hardness, self-lubricating
properties, dimensional stability, and excellent thermal and electrical conductivity.
As such, zinc die-cast alloys are the second most common first-use of zinc globally, according
to the ILZSG. Recent advances in technology have also led to new zinc-based materials, such as
zinc foams, that can be die-cast in ordinary die-casting machines at the same price as
conventional zinc die-castings, but result in parts that are approximately 30% to 50% lighter.
Brass is an alloy of copper and zinc, usually made up of 65% copper and 35% zinc, though the
exact mix depends on the desired properties of the alloy. Brass is cheap and easy to produce
but attracts a high price as it can be cast, forged, and formed into sheets, wires and rods.
Due to its high tensile and yield strength, brass is also machineable and therefore can be used
to produce complex shapes. Brass is widely used in hydraulic and electrical components due to
its conductivity, non-magnetic propensity and corrosion resistance. It is also used widely in
the plumbing industry to create complex and durable pipes and joints, as well as in the
automotive industry as a component of fuel, electric and braking systems.
Zinc is also used in chemicals, such as zinc oxide. According to the IZA, zinc oxide is
primarily used as a vulcanizing activator in the rubber industry, where it is critical to the
production of tires. Zinc oxide is also widely used in ceramics, where it is used to produce
frits, artistic glasses and enamels. Other uses for zinc oxide include as a pigment in paints
and as an ingredient in sunscreen, baby creams and wound-care ointments. One potential future
growth area for the use of zinc is as a micro-nutrient in fertilizers. According to the IZA,
research shows that the addition of zinc can lead to a significant boost in crop yields, which
has the potential to dramatically increase zinc consumption if widely adopted.
According to the IZA, the final primary first-use for zinc is in semi-manufacturing of rolled
and extruded products. These products are in turn used in a variety of forms across the globe.
For example, zinc sheets are commonly used in the building industry as roofing and cladding
material, as well as coinage and as battery cans. According to IZA, the largest end-use sector
for zinc is construction, with galvanized steel being used extensively in various
infrastructure projects including bridges, electricity transmission towers and lighting poles.
According to the IZA, the second most important end-use sector is transport, followed by
consumer products and the manufacturing of industrial goods and equipment. In the 2016 edition
of its capital projects and infrastructure spending report, PricewaterhouseCoopers LLC.
forecasted global infrastructure spending to grow at a rate above 4% per annum from 2017 to 2020.
Zinc Smelting
The most common process used for smelting and refining zinc is hydrometallurgical, commonly
referred to as the electrolytic process or RLE. According to the IZA, this technique accounts
for over 90% of all concentrate treated globally. The other technique in use is pyrometallurgical,
which includes blast furnace, electrothermic and vertical retort smelting and refining.
Smelters and miners have ongoing relationships (where not vertically integrated) and will
continuously negotiate private contracts at a variety of rates. Smelters purchase concentrate
under annual or multi-year tonnage based contracts, but will also purchase concentrate on the
spot market in order to fill their plants should supply be limited. Smelters may also treat
secondary feed materials such as zinc oxides. Based on the public filings of major smelting
operations, under a typical commercial smelting contract, the smelter pays the seller for 85%
of zinc contained in concentrate and receives any metal above that recovery, known as free metal.
Treatment charges are paid by the concentrate seller as a charge for the treatment of the zinc
concentrates, typically quoted in United States dollars per tonne of concentrate. The contract
between the smelter and the seller typically provides a base treatment charge at a base zinc
price that will escalate and de-escalate as a result of movements in the zinc price over the
quotation period, effectively acting as a form of price participation by smelters. These terms
are negotiated between the smelter and seller and will fluctuate year to year. In a given year,
treatment charges will generally settle around precedents established by major buyers and
sellers of concentrate at terms referred to as the benchmark treatment charge. A strong view on
supply and demand over a one year time horizon, and therefore zinc price, is essential to these
negotiations. A spot treatment charge exists for the sale and purchase of certain parcels of
concentrate that become available to the market during the course of a year and can provide an
indication of the supply and demand balance for zinc concentrates at a specific point in time.
The market for spot treatment charges is much less liquid than the one that exists for
benchmark treatment charges.
When the supply of concentrates is low, miners gain bargaining power on pricing. This is currently
evidenced by the loss of price participation for zinc smelters, which is a mechanism that
allows smelters to see upside when zinc prices move above a basis price and mitigates downside
risk to miners if it goes below that basis price. While price participation was not explicitly
removed from contracts, price escalators and de-escalators have been set at zero for 2017, an
outcome which miners have been negotiating for a long time.
Zinc Price History
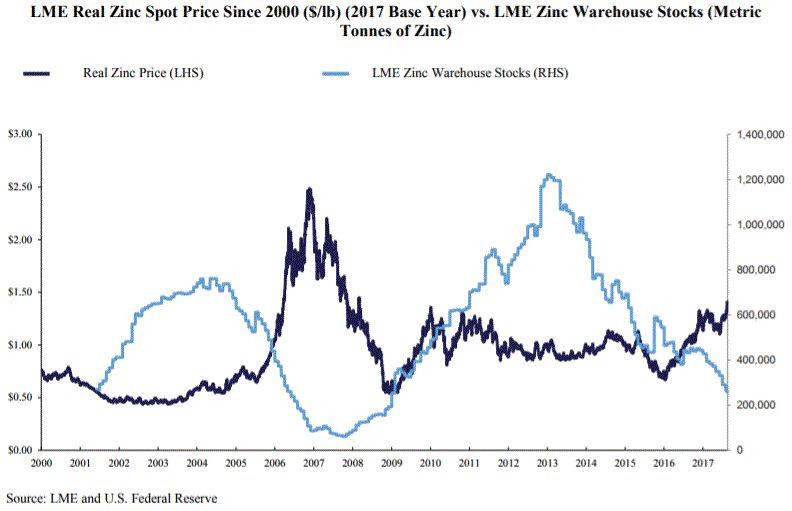
Over the past five years, the LME spot zinc price has ranged from a low of $0.66/lb to a high
of $1.41/lb. This range is significantly below prices observed in 2006, which peaked at $2.09/lb
on November 24, 2006, and which suggests that there is room for price appreciation should
fundamentals support a price increase. As a base metal, zinc prices are much more driven by
supply and demand fundamentals than trading speculation. Thus, the decline of global zinc
inventories over the past few years, which effectively tightened supply, could be perceived as
a contributing factor to the rally in zinc prices since the beginning of 2016. Historically,
the price of zinc has moved inversely compared to inventory levels and has displayed significant
upward increases when inventories reach critical levels, as witnessed in 2006-2007.
The two main sources of zinc pricing are the LME and the SHFE. The LME official cash price is
set through open outcry trading on the floor of the LME. Each metal trades in five minute ring
sessions which are said to be representative of global supply and demand. The last bid and
offer price quote during the second ring trading session for zinc metal is set as the LME
official cash price for zinc for that particular trading day. The SHFE price is representative
of the supply/demand balance in China as it is the typical exchange for Chinese commercial
transaction.